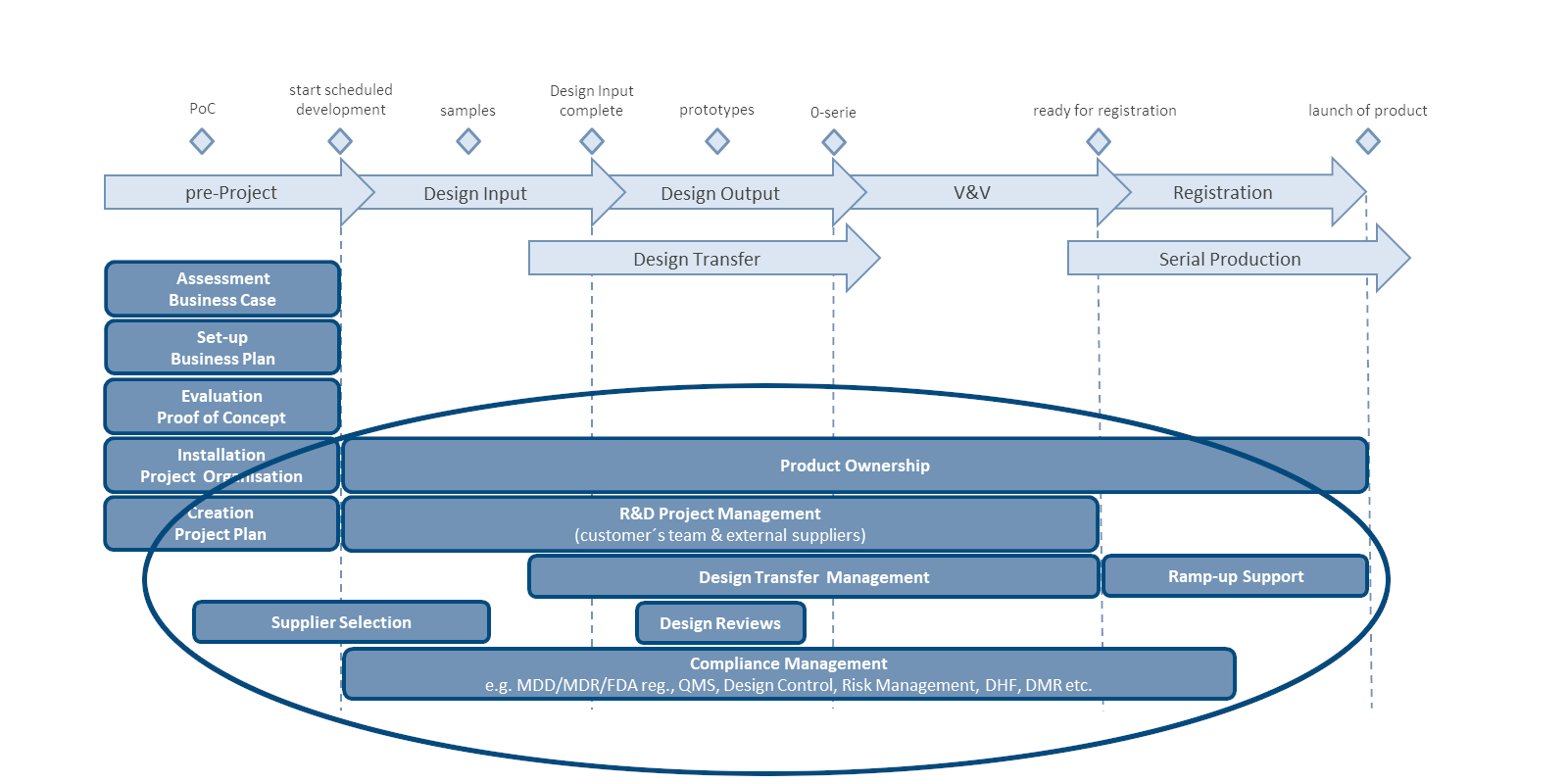
Custom-tailored to your specific medical device or combination product.
We take your team and external suppliers through the entire product development process all the way to market launch – including design transfer.
To ensure successful registration (CE, FDA etc.), we ensure that all regulatory requirements are taken into consideration.
We coordinate all activities on the basis of a detailed project plan. We continuously track completion status and, if necessary, implement corrective measures.
We conduct the supplier selection process, including formal requests for proposals, evaluations, agreements, and follow-up.
We keep an eye on project risks. We proactively develop countermeasures and take measures to implement them.
We are fully committed to the project goal and provide our customers with regular, personalized reports.